04.6.13.14 openchat-3.5-0106
Model Description
The @cf/openchat/openchat-3.5-0106 model includes two nodes:
- openchat-3.5-0106 Prompt (preview)
- openchat-3.5-0106 With History (preview)
note
Model ID: @cf/openchat/openchat-3.5-0106. OpenChat is an innovative open source library of language models customized with C-RLFT, a strategy inspired by autonomous reinforcement learning.
The model can be used for a wide variety of natural language processing tasks. Here are some of the main applications:
- Text generation - the model can generate coherent, cohesive texts on a given topic or by a given sentence start. This is used for writing articles, essays, short stories, etc.
- Question answering - by asking the model a question, a relevant and informative answer synthesized from the model's knowledge can be obtained.
- Paraphrasing and summarizing - the model can paraphrase a text in other words, and highlight and summarize the key points of a long text.
- Translation - the model can be trained on translations between languages for automatic machine translation.
- Tonality and sentiment analysis - the model is able to detect the tone and emotional coloring of texts.
- Dialogue maintenance - the model is able to maintain coherent dialogs by interpreting the context and responding with appropriate rejoinders.
Example of launching a node
A description of the node fields can be found here.
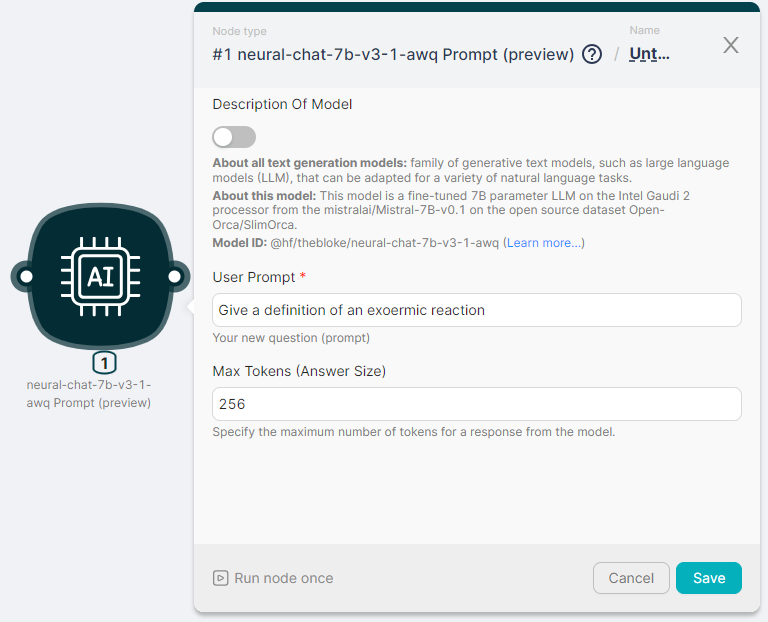
Let's start the openchat-3.5-0106 Prompt (preview) node to process the text and generate a response with parameters:
- User Prompt - Give a definition of an exoermic reaction;
- Max Tokens (Answer Size) - 256.
The output of the node execution is JSON:
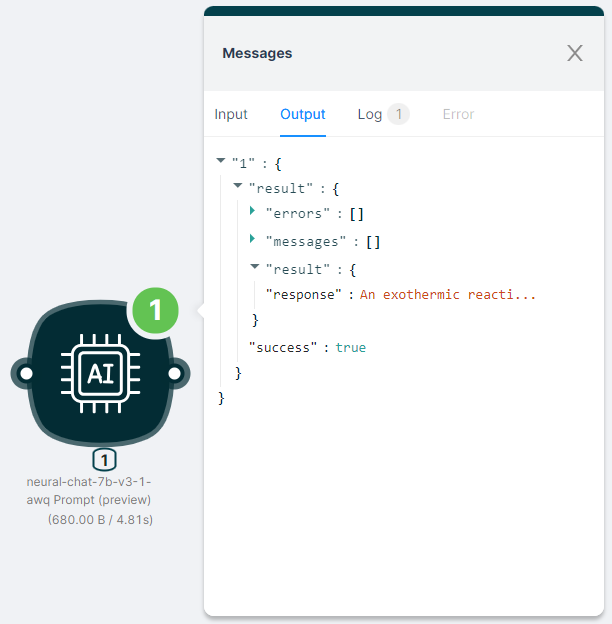
- with a response to the
"response"request;
- with the status of the action
"success": true.
JSON
{
"result": {
"errors": [],
"messages": [],
"result": {
"response": "An exothermic reaction is a chemical process in which the total energy released during the reaction is greater than the energy required to initiate it. In simpler terms, it is a reaction that produces more energy than it consumes. This excess energy is often released as heat, light, or other forms of energy. Examples of exothermic reactions include burning fuels, such as combustion of wood or gasoline, and the decomposition of certain organic compounds. These reactions can be used for various purposes, such as generating electricity, cooking food, or producing heat for warmth."
},
"success": true
}
}